Intermolecular Potential Energy
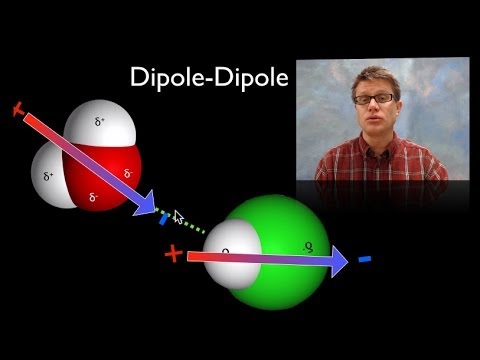
In this video Paul Andersen explains the importance of intermolecular forces in chemistry. Intermolecular forces exist between dipoles (like hydrogen bonds), between dipoles and induced dipoles (like Ar and HCl) and between induced dipoles. The energy of the force is based on the size of the molecule and the number of electrons.
Bozemanscience Resources
Intermolecular Potential Energy Concept Map
Intermolecular Potential Energy Slideshow